Product
COVID-19 Rapid Test for Antibody
COVID-19 Rapid Test
for Antibody
Wantai's rapid SARS-CoV-2 total antibody test is designed for evaluations of patients suspected of infection with the SARS-CoV-2 Virus, an aid in the diagnosis of the COVID-19 disease.
The Most Fast Test
Delivered serology tests (August 28,2020)
01. Test Preparation
Allow the test cassette to reach room temperature, use it within 20 minutes after opening the bag.
02. Add the sample
Add 10 ul of whole blood, serum or plasma samples. Add two drops of diluent buffer.
03. Read the Result
Read the results in 15 min.
Advanced Diagnostic Technology
Along with the spreading global pandemic of Novel Coronavirus, countries around the world have been struggling to diagnose and control this disease in time. Xiamen University and Wantai have jointly developed a series of innovative, highly sensitive and specific serological and molecular assays for testing of Covid-19.
01. Lateral flow test: suitable for rapid on-site testing
02. Performance: sensitivity of 94,70% (125/132) and specificity of 98,89% (268/271). The test have been clinically validated during the C0VID-19 / 2020 in China
03. Easy to use: without special equipment. Works with samples whole blood, serum or plasma
04. Fast: clear answer in just 15 minutes
Wantai SARS-CoV-2 Diagnostic Kits:
IMPORTANT: THIS PRODUCT IS INTENDED FOR PROFESSIONAL USE ONLY, NOT FOR SELF-TESTING OR TESTING AT HOME
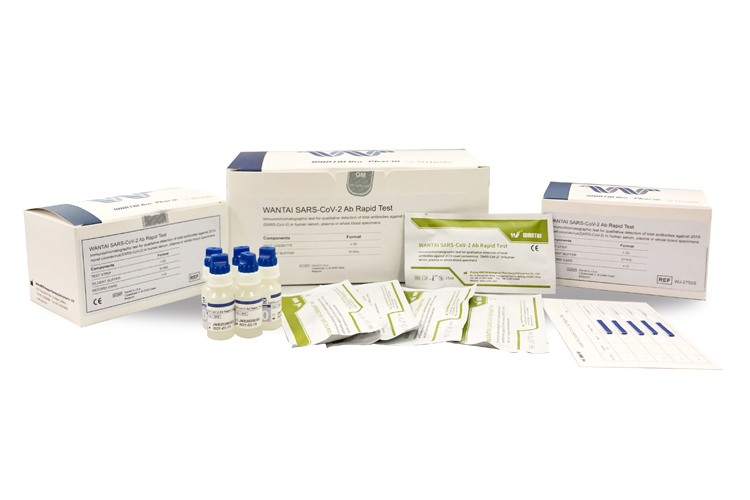
Rapid Test for Total Antibody to SARS-CoV-2
IMPORTANT: THIS PRODUCT IS INTENDED FOR PROFESSIONAL USE ONLY, NOT FOR SELF-TESTING OR TESTING AT HOME!
WANTAI SARS-CoV-2 Ab Rapid Test is a 15minutes test that detects total antibody as indicative of an immune response to SARS-CoV-2 in patients suspected of previous SARS-CoV-2 infection, or for the detection of seroconversion in patients following known recent SARS-CoV-2 infection. The kit is based on double antigen "sandwich" method and it detects TOTAL ANTIBODIES against S-RBD. The test may also be used to aid in the diagnosis of acute or past SARS-CoV-2 infection in conjunction with other tests and clinical information. The prevalence of SARS-CoV-2 infection in the area where testing has occurred should be considered when interpreting positive test results. The test should not be used as the sole basis for diagnosis.
Performance evaluations (click on the links to open each study report):
In a limited validations, the RIVM National Institute for Public Health in the Netherlands, and the Ministry of Health in the Czech Republic both reported sensitivity and specificity of 100%. In Austria, the Medizinische Universität Wien calculated sensitivity of 80% (6-10days) and 100% (>11days) respectively, and the University of Innsbruck reported sensitivity of 95.7% (100% >14days) and specificity of 100%. In France, the French National Reference Center (CNR) evaluated the kit and conclude that it has sensitivity of 89% (7-13days), 92% (14-19days) and 94% (>20days) respectively.
In the United States, validation study conducted by the National Cancer Institute (NCI) showed sensitivity of 100% (30/30) and specificity of 98.8% (79/80) of the test. Subsequently, WANTAI SARS-CoV-2 Ab Rapid Test received FDA Emergency Use Authorization on July.10,2020. Downloads: Letter of Authorization , HCP , Recipients , IFU , NCI Report.
FIND evaluation results are expected soon.
Additional Information: Fact Sheet HCP Fact Sheet for Recipients