Product
COVID-19 Nucleic Acid Diagnostic Kit
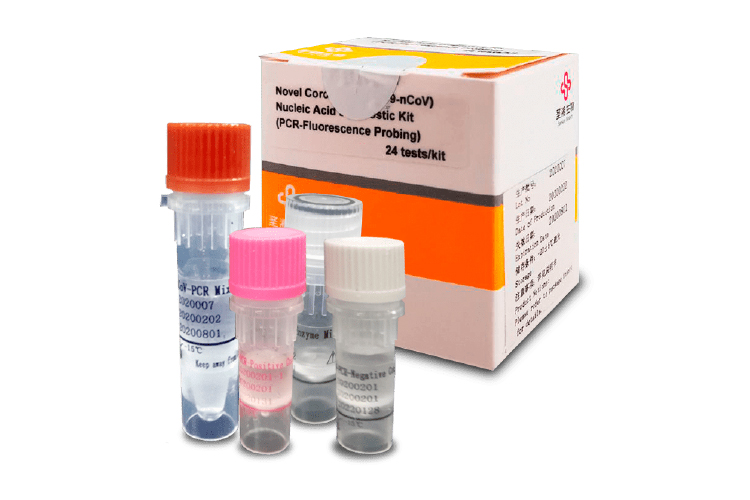
COVID-19 Nucleic Acid Diagnostic Kit
CE-IVD, NMPA, FDA-EUA, Brazil ANVISA etc.
The kit manufactured by Sansure Biotech is used for the qualitative detection of the ORF1ab and N genes of the novel Coronavirus (2019-nCoV), from samples that have been obtained from nasopharyngeal and oropharyngeal exudate, alveolar lavage fluids, sputum, blood plasma, blood total, and stool from patients with suspected atypical pneumonia.
Summary:
| Regular Method | Simplified Method |
|---|
| FDA approved Viral RNA Extraction Kit can be used as alternative extraction method | Fast and simplified One-step nucleic acid extraction kit free technology. No boiling, no tube change; nucleic acid extraction can be accomplished by a simple operation. By one simple step of centrifugation and lysis, the sample mixture can be directly added to the 2019-nCoV-PCR master mix (2019-nCoV-PCR Mix + 2019-nCoV-PCR-Enzyme Mix) to carry out rRT-PCR amplification |
| Item | Specifications |
|---|
| Size | 24 Test x Kit. 48 Test x Kit |
| Intended use | The Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from SARS- CoV-2 in nasopharyngeal swabs, oropharyngeal (throat) swabs, anterior nasal swabs, mid- turbinate swabs, nasal washes and nasal aspirates from individuals who are suspected of COVID- 19 by their healthcare provider |
| Storage | Diagnostic kit: -20 ±5°C
Sample Storage Reagent 2 - 8°C or below |
| General Description | This kit is used for the qualitative detection of the ORF1ab and N genes of SARS-CoV-2 RNA. By one simple step of centrifugation and lysis, the sample mixture can be directly added to the 2019-nCoV-PCR master mix (2019-nCoV-PCR Mix + 2019-nCoV-PCR-Enzyme Mix) to carry out rRT-PCR amplification. QIAamp Viral RNA Mini Kit (50, Catalogue No. 52904) can be used as an alternative extraction method. Internal control targeting the RNase P gene monitor the sample collection, sample handling, and rRT-PCR process to avoid false-negative results. The LoD of the kit is 200 copies/mL |
- • Sensitivity: 200 copies/ml
- • Internal control targeting the RNase P gene monitor the sample collection, sample handling, and rRT-PCR process to avoid false-negative results
- • Quick results
- • IFU-COVID-19 Nucleic Acid Diagnostic Ki
Download Fact Sheets:
Fact Sheet HCPFact Sheet for Patients
01.
Rapid detection that allows the isolation of patients.
02.
Evidence for clinical diagnosis. Monitor the virus.
03.
Provides reliable data for epidemiological surveillance.
Advanced Diagnostic Technology
The dual-target gene design is based on the 2019-nCoV ORF1ab target regions and the conserved specific sequence encoding the nucleocapsid protein N gene. Detects 2019-nCoV RNA virus in real time by PCR system with PCR mix.
Required laboratory equipment:
- Thermal cycler
- Laboratory centrifuge with rotor for microtubes and plate
- Laminar flow hood
- Vortex shaker
- Micropipettes
- All these equipment are also available through us. Contact us for more information.
COVID-19 Nucleic Acid Diagnostic Kit:
IMPORTANT: THIS PRODUCT IS INTENDED FOR PROFESSIONAL USE ONLY, NOT FOR SELF-TESTING OR TESTING AT HOME
Advantages
- One-tube technology, extraction within 30 minutes
- Up to 96 samples at one time
- Simple operation process, no need long term staff training
- Room temperature nucleic acid lysis, No heating
- Direct sample types include: Nasal, Throat, and Nasopharyngeal swabs
- Enhance screening efficiency
Parameters
- Technology: One-tube fast test/ Mag-beads
- Sensitivity: 200copies/mL
- Lysis: Room temperature lysis
- Sample types: Alveolar lavage fluid; throat swab, sputum (varys in different registration version)
Additional Information: Fact Sheet HCP Fact Sheet for Recipients